Specific Gravity of Gemstones
Archimedes is said to have discovered that when a body is placed in water the volume of water displaced is equal to the volume of the body and that when the body is placed in water that it undergoes an apparent loss of weight. This loss of weight is equal to the weight of the water displaced. When a gem is weighed in air and then weighed in water the loss of weight is equal to.
10 Minute Read
Archimedes is said to have discovered that when a body is placed in water the volume of water displaced is equal to the volume of the body and that when the body is placed in water that it undergoes an apparent loss of weight. This loss of weight is equal to the weight of the water displaced.
When a gem is weighed in air and then weighed in water the loss of weight is equal to the weight of its volume in water displaced. The weight of the gem in air divided by the loss of weight in water gives the specific gravity of the stone or material.
Specific gravity is then defined as "The weight of a body compared with the weight of an equal amount of pure water at 4oC (4oC is essentially the temperature at which water is densest). In practice room temperature water is used. What this means is that a given volume of ruby weights 4 times as much as an equal volume of water or a given volume of diamond weighs 3.52 times as much as an equal volume of water.
An example of using this is: gem weighs 5 ct in air, loss of weight is 1.25 ct. in water (weight units do not matter, grams, carats).
Calculate:
The gem is then corundum with an SG of 4.00 (3.99).
The specific gravity for each mineral is the same within narrow limits (except in species like topaz or garnet and zircon where subgroups with definite specific gravities occur). If one can determine the specific gravity of a gem one can usually identify it. In practice however because of time constraints and other factors specific gravity determination is usually used as a corroborative test or for large, rough or carved samples.
There are a number of SG balances made for measuring specific gravity. These allow the SG of the sample to be read directly from their scales. Two are the Westphal and the Hanneman. The Hanneman balance is extremely inexpensive ($10.00 US), fairly fast and easy to use. It is very accurate and can give accurate results on stones as small as .5 ct with care while other balances are not accurate below 3.0 cts. There are 5 cts to the gram.
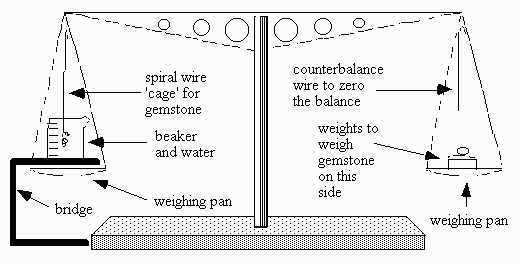
A regular diamond or chemical balance, mechanical or electronic may be adapted for hydrostatic SG determinations by devising a way of weighing the stone in air, then in water, using the same pan. The conditions required are:
1. A bridge is constructed for the left hand side of the balance. This sits above the pan without touching or otherwise interfering with the operation of the balance at any time in its use. The bridge should not be magnetized.
2. A beaker of water (distilled, deionized, degassed) is placed on the bridge. A tiny drop of wetting agent, alcohol, or better photo-flo or dishwashing liquid is added to the water to decrease surface tension which can adversely affect the results. This too may not hinder or touch the operation of the balance.
3. An extremely thin lightweight metal spiral wire 'cage' is constructed which hangs from the left hand side of the balance arm. The spiral 'cage' is submerged in water. It should be of rustproof metal like brass, copper, stainless steel, titanium or tungsten.
4. A counterbalance wire is hung from the opposite side of the balance arm at the same position. This is cut so as to balance the 'cage' wire as completely as possible
diamond or chemical balance accurate to 3 decimal places (.001g)
Procedure to Determine SG of a Gemstone Over 3 ct
- Zero the balance. If your counterbalance wire does not do this you have to add some weights to the right hand pan (for right handed people) to get the indicator needle of the balance to register zero or perfect balance. This weight must of course then be subtracted from your later additions of weights to this pan.
- Clean the stone very carefully with a gem cloth. If it has wax or grease on it this must be removed. Use alcohol and wipe clean. Examine it for large flaws or inclusions which might affect the accuracy of the results.
- Place it on the left hand pan underneath the bridge and weight the stone. Record the result. Weighings are done to three decimal places.
- Place the stone in the spiral cage and weigh it in water. Record the result. Brush off any bubbles on the stone or wire with a fine brush.
- Subtract the second result from the first to find the difference. (The loss of weight in water).
- Calculate:
- For more accurate results repeat the procedure at least three times and average the results. Make sure the stone is dry before repeating the air weighing.
With an electronic balance the procedure is similar except that only one balance pan is used. As you will not be able to zero the balance the weight of the spiral cage suspended in water is the zero weight and all calculations are done from this reference point. eg. if it weighs .3 grams then subtract .3 grams from all calculations.
There are several other methods of determining specific gravity hydrostatically. For large specimens a spring balance and a bucket of water may be used. The larger the object the more accurate the results.
Other Balance Methods
(Sinkakas, Gemstone and Mineral Databook, pp 123 -126)
For large samples, sculptures, rough.
- Weigh specimen in grams.
- Partly fill a graduated cylinder (cc) with water.
- Drop in sample, note new volume.
- Difference between volumes = volume of water displaced in grams.
- Calculate:
Also:
- Weigh specimen.
- Place a beaker partly filled with water on balance and weigh.
- Leaving beaker and water in place submerge the sample with a very thin thread or nylon monofilament in the water.
- The beaker and water gain weight, rebalance and note new reading.
- The difference between new weight and earlier weight is the weight of the water displaced by the sample. This too is a rough method.
Causes of Error in Hydrostatic SG Determination
- Too small a specimen.
- Surface tension of water. One drop of detergent liquid the size of a pin head destroys surface tension in 1 liter of water.
- Failure to degas water. Use boiled, deionized water that has been sitting for a time.
- Failure to record and compensate for temperature corrections when using liquids other than water.
- Failure to multiply SG by density of liquid other than water.
To avoid problems of surface tension other liquids than water may be used. Examples are Carbon Tetrachloride, toluene and alcohol. Ethylene dibromide was used but is now considered too dangerous. Recommended is toluene or alcohol. Because temperature affects the SG of these liquids one must refer to the literature for each temperature change when using them. In practice it may be better to find the exact SG of the liquid you are using at the time of use and then refer to your SG and temperature correlations as you build them up with time. Such liquids are used for small stones to increase accuracy. To find the SG of a liquid use a very clean piece of quartz which has a constant SG of 2.651 (a gemstone and weigh as if to obtain the SG. Then solve the equation:
Heavy Liquid Methods
Various liquids and chemicals have a wide range of SGs. When a stone is placed in a liquid of the same SG it suspends, and stays where it was placed (or sinks or rises very slowly). If it has a lower SG (is 'lighter') than the liquid it floats. If it is denser (has a higher SG, is 'heavier') it sinks. Therefore, given a range of liquids of known SGs it is possible to estimate or even determine the SG of a stone by its behavior in the liquids.
Heavy liquids should not be used at all without a properly tested fume hood. Cleanliness and proper working methods are essential because they can cause severe health damage and can be absorbed through the skin and by breathing. They belong in a properly equipped gemmolocial laboratory.
Advantages of Heavy Liquids Include:
- Speed. It is very fast. One usually begins with the densest liquid and goes to the next less dense until the stone sinks to the bottom. If you are lucky it suspends.
- One can use them for stones under 3 ct. Stone size makes no difference.
- As an ancillary method it may be very rapid to check or corroborate other tests.
- Very quick for separating different types of similar appearing stones from the same package. For example checking beryl (emerald) one might make up a liquid with the density of 2.71 (Indicator is calcite).
Disadvantages of Heavy Liquids:
- The liquids used are really toxic, hazardous to your health, messy, smelly, poisonous and in some cases corrosive.
- Cracked, flawed stones may give inaccurate readings.
- Stones must be unmounted.
- Porous stones may not be tested (opal, turquoise, organic gemstones).
- The liquids attack many plastics.
There are three liquids recommended by gemology text books for general use.
Bromoform SG 2.88
Methylene Iodide SG 3.33
Monobromonapthalene SG 1.49
This is used to dilute and lower the SGs of the other two. Liddicoat suggests the use of toluene which is very flammable and evaporates faster than monobromonapthalene but is cheaper.
| Liquid Number | Indicator | ||
| #1 | 2.65 SG | Bromoform diluted with monobromonapthalene. Quartz, feldspars, iolite float, most other stones sink. | Quartz |
| #2 | 2.88 SG | Bromoform, undiluted. Beryl floats, other green blue stones sink. | |
| #3 | 3.05 SG | Methylene iodide diluted with monobromonap- Tourmaline thalene. Tourmaline floats, nephrite floats, jadeite sinks. | |
| #4 | 3.33 SG | Methylene iodide undiluted. Jadeite, peridot suspend or float or sink very slowly, topaz sinks, tourmaline floats, etc. |
There are also solutions made up with an SG of 3.52 (diamond indicator) and 4.00 (corundum indicator) using a liquid called Clerici solution which may be diluted with water.
Indicators of clear gemstones may be used as a check on the liquid SG before use as evaporation etc. can change the SG. Manufactured glass SG indicators in a wide range are also available. It should be noted that relative speed of sinking is a good indication of SG range. If a stone sinks very rapidly then it is a lot denser than the liquid, slowly it is similar. The degree to which it floats on a liquid can also tell something about its SG. High floating means it is a lot less dense, low floating similar, suspends - the same. Make sure to tap or dunk a stone with tweezers to ensure that surface tension is not holding it up.
Although Clerici solution offers many advantages to the gem tester it is no longer recommended as it is extremely toxic, corrosive and recently shown to be carcinogenic. It demonstrates an exact correlation between SG and RI as it is diluted. It may be diluted until a gemstone suspends and then a drop of it placed on a refractometer and the RI found. The one looks at a straight line graph and reads off the SG of the solution and the stone. To reconcentrate it water is simply allowed to evaporate. At full concentration its SG is 4.28. The less toxic liquids can also be used in the same way to obtain a correlation between SG and RI. However they are not easy to reconcentrate. There are also other methods of obtaining the SG of a stone using heavy liquids.
Using Heavy Liquids
- Adequate ventilation is necessary.
- All tweezers, stones, etc. in contact with the liquids must be very carefully cleaned; perhaps with toluene as a solvent; between liquids to prevent contamination or corrosion of tools. Clean them extra carefully after finishing.
- They are poison. No food, smoking or drinking is allowed when using heavy liquids.
- Hands must be washed right after use whether or not they were in contact with the liquids.
The Hanneman Balance
To use the Hanneman Balance: (A small amount of detergent in the water will increase accuracy)
- Set up balance as described.
- Clean the stone, check for flaws.
- Slide zero weight to a position where the indicator needle registers zero on the zero card. If necessary adjust the zero card slightly. The balance should be as horizontal as possible.
- Place stone in top left hand pan. Place indicator weight wires on the notch at the right of the balance until the needle registers zero again.
- Place the stone carefully in the lower pan under water. Brush off any air bubbles. The needle will rise as the weight is less.
- Slide the indicator weights wire to the left on the scale until the needle registers zero again.
- Read the SG value (top scale). Repeat twice more and average for increased accuracy.
You can order the Hanneman balance and other items from: Hanneman Gemological Instruments, PO box 942L, Poulsbo, WA, 98370, USA
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
Charles Lewton-Brain
Master goldsmith Charles Lewton-Brain trained, studied and worked in Germany, Canada and the United States to learn the skills he uses. Charles Lewton-Brain is one of the original creators of Ganoksin.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.