Platinum Casting Investments
In this paper I will attempt to give some insight into the investments used to cast Platinum and also talk about how we go about developing them. While this particular class of materials has been around for a long time, there have been recent improvements brought on at least partially by the increased interest in Platinum jewelry. I will briefly consider some of the properties which we seek in an investment and comment on the differences between gypsum and phosphate bonded investments on the one hand and the Platinum investments on the other.
19 Minute Read
In this paper I will attempt to give some insight into the investments used to cast Platinum and also talk about how we go about developing them. While this particular class of materials has been around for a long time, there have been recent improvements brought on at least partially by the increased interest in Platinum jewelry.
I will briefly consider some of the properties which we seek in an investment and comment on the differences between gypsum and phosphate bonded investments on the one hand and the Platinum investments on the other. I will then go into the variations of Platinum investment of which I am aware and discuss how we test them. This will include a description of some of their properties. Finally, I will try to relate our understanding of how the materials work to how they are used.
Investment Types
When considering the type of maid material that can be used to cast a specific metal it is necessary to first consider the properties of the metal being cast. With Platinum and its alloys the most important of these are high melting point and high specific gravity. Table 1 compares these properties with those of other alloys used in jewelry.
Due to their high melting points, Platinum alloys have to be transferred rapidly into the mold, and this is generally achieved with high speed centrifugal casting. This, coupled with the high specific gravity, places a considerable force on the investment. As there is a need to get the metal into the cavity rapidly, there is also a need to get the air out rapidly. The permeability of the investment is therefore of interest.
| Table 1: Properties of various metals. |
Not all about casting Platinum alloys is negative however. Platinum and its alloys, in particular these with other precious metals, are relatively chemically inert, and are thus less likely to attack the investment.
As most people are more familiar with gypsum-bonded investments, I will talk briefly about them and then compare the Platinum investments to them.
Gypsum investments are generally composed of 25-30% CaSO4.½H20, 70-75% silica and 1% additives which control the setting behavior, fluidity, wetting behavior and vacuum rise of the investment. It is a fairly complex chemical system. Standard Gypsum investments contain eight chemically different components.
They set by a chemical reaction between the binder and water:
This gives a distribution of fine silica particles held together by the gypsum. Two forms of silica are used, quartz sand and cristobalite. During burnout the CaSO4.2H2O loses the water and would shrink and crumble if not for the presence of the cristobalite which, in the temperature range where the gypsum is losing its water, goes through a phase change which involves considerable expansion. This more than compensates for the shrinkage of the gypsum and the total system holds together because it goes into compression within the flask. When you mix a gypsum investment, the amount of water that is used is much greater than that needed for the chemical bonding reaction, and its function is to give a workable mixture. The excess water departs in the early stages of burnout.
There is another somewhat similar system which is used to a limited extent in jewelry manufacturing. This is the phosphate system. Its setting reaction can be described as
2(NH4)H2PO4+ 3MgO = Mg3(PO4)2+â
It is used to cast non precious metal school rings and has much higher temperature stability than gypsum. It is also used in the dental lab to work with higher melting point metals. I know that people have tried it with Platinum but I have not heard of anyone having much success.
The investments used with Platinum are chemically much simpler than those that I have just described. They are basically about 99% fine silica with up to 1% additives. The silica is all quartz with no cristobalite. The additives serve very different purposes than those used in the other investments and may be either in the powder or in the liquid used with the powder. The liquid itself may be water or a dilute acid, generally Phosphoric.
The mold that results from using platinum investments depends, for its structure, on both what happens when it is mixed and allowed to set and also on changes as it is burnt out. The additives and the dilute acid can have an effect in both stages. Initially they allow the particles of sand to be uniformly dispersed in the liquid at a low liquid/powder ratio. They also participate in the formation of a gel structure which eventually sets to give the structure, which provides green strength. During burnout they react with the silica to form mixed oxides which bond the particles to each other.
I have mentioned that diluted phosphoric acid can be used as the liquid. Another way to go is to add certain relatively weak acids which are available as powders to the sand and use water as the liquid. Among the powdered acids that we have evaluated are Silicic acid (H2SiO3) as well as the organic acids, such as Oxalic, Malaeic and Lactic.
The most commonly used sintering agent was Sodium Silicate. This itself can be considered as a mixed oxide of sodium and silicon. Phosphoric acid is essentially a solution of Phosphorous oxide in water and therefore also has the capability to form mixed oxides.
In the simplest of these materials we do not use deliberate acid additions, although the sintering agent which we do use performs some of the same functions that a deliberately added acid would in terms of making the mixed investment more fluid. This material, which I will denote as A, works with water at a ratio of about 34/100. It is relatively difficult to incorporate and when it is mixed the practice is to add about three quarters of the powder to the water, Incorporate it by hand, start mechanical mixing and then slowly add the rest of the powder. For a small mix (4-6 lbs.) it requires as long as 12 minutes to establish the required structure in the slurry and larger mixes take much longer (up to 20mins). This material partially sets by settling, which means that as it sets the sand particles can move around and take up their final position. This material needs up to 16 hours to set and at the end of setting there is a layer of water on the surface which is decanted off. It can be used under conditions where some of the water is wicked off during settling or it can be used with a rubber base like a gypsum.
At the other end of the range are materials which use diluted phosphoric acid as the liquid and which use a sintering agent which gives a more refractory mixed oxide than does sodium silicate. I will describe this material as C. It is mixed at a liquid powder ratio of 30/1 00. It does not show the settling behavior of the A type and its structure is basically established during the mixing process. It is always used with a system which allows the removal of water from the investment and requires a shorter setting time than the A type. This material uses a finer sand than does the A material.
Intermediate between the A and C type is one which I will call B. It is similar to A except that it contains a small amount of a powdered acid. This makes its incorporation somewhat easier. There is some settling during its setting process but not to the extent to which the A material settles. It should also be used in such a way that water can be wicked out of it.
As you can see, there is no well defined chemical reaction occurring in these materials as there is in the Gypsum and Phosphate bonded materials. There are some weaker electro-chemical interactions occurring between the silica particles and between these particles and their liquid environment. These lead to gelling and this gives the material sufficient green strength to allow it to be handled.
Test Procedures
I would now like to describe how we test these materials. The tests which we carry out are:
- Fluidity (Zahn Cup)
- Permeability
- Fired Strength
- Thermal Expansion
- Casting
- Particle Size
Zahn Cup
This is a test which is covered by ASTM standard D4212 "Standard Test Method for Viscosity by Dip Type Viscosity Cups." Its intended use is with paints, varnishes, lacquers and similar materials. Basically, a U-shaped container with a hole in the bottom is immersed in the fluid being tested and then lifted out so that the fluid can flow out through the hole. The time for the fluid to flow from the cup is measured. The cups are designated as #1 through #5 based on the size of the hole, and we have used both the #4 and #5 cups in developmental work. These have the largest holes and we now think that the #5 is the appropriate choice.
Permeability
We run this test with an Automated Gas Permeameter made by a company called Porous Materials Incorporated. The specimen is prepared by filling a steel ring which is two inches in diameter and one-half inch thick with Investment and allowing it to set. The ring has a collar to allow over-filling and this is removed. The two surfaces of the investment are then trimmed flat and level with the ends of the ring and the ring and Investment are burned out at the temperature of interest. We generally use 1600F as the burnout temperature but other temperatures are used as seems appropriate.
To measure the permeability, the equipment places air pressure across the material and measures the gas flow as the pressure is slowly increased. From this it calculates a value of the relative permeability of the material based on the rate of gas flow.
Fired Strength
Plastic molds with a diameter of 20mm and a length of 40mm are used to produce cylindrical specimens in much the same manner as the metal ring was used for the permeability specimens. The maids are removed prior to burnout and the specimens tested by loading them axially in an Instron testing machine.
Thermal Expansion
Cylindrical specimens 1½" long by ½" diameter are produced using a steel mold in much the same manner as above. They are heated at a rate of 5°C/minute in a Theta dilatometer. Above is a typical Thermal expansion curve.
Casting
Ideally we would like to be able to cast all of our experimental materials with Platinum or Platinum alloys. Unfortunately this is cost prohibitive. What we actually use are either steel or super alloys. To maximize the stress on the investment we use a range of patterns including relatively difficult to fill thin ones and very large bulky men's rings. The flasks are either 3×5 or 4×6 and the patterns are spurred as trees. We use a centrifugal casting machine with a high speed to maximize stress. When we have a high degree of confidence that we have developed a product as far as we can, arrangements are made by our marketing people to have the materials evaluated by well-established Platinum casting facilities who give us feedback and we proceed from there. We greatly appreciate this feedback.
| Fig. 1: Typical Thermal Expansion Curve |
Particle Size
These investments are 99% silica and the particle size of the silica particles is one of the most important properties. We measure the particle size of both the sand and the investment using a piece of equipment called a sedigraph. The particular unit which we use is the Sedigraph 5100 produced by micromeritics. This equipment evaluates the rate at which sand particles sink through a liquid, considers the sand particles to be spherical in nature and calculates an equivalent diameter. It then presents the size distribution of the particles as a graph .We pick off a specific size (10 micron) and note the % of the particles which are smaller than this. This is a good way of evaluating a specific sand for code to code variations or looking at different sands which come from the same mine and grinding equipment. When sands have been ground differently or come from different sources, direct comparisons are more difficult to make as the precise particle shape takes on great importance. As a word of warning, there are different types of measuring equipment that can be used to measure particle size and these will give very different answers on the same material. If you compare the particle size of two sands, you need to make sure that the same equipment was used.
Product Development
First I would like to go over the use of the Zahn Cup test. This is a very simple and useful test to allow us to both understand what is happening in the investment and also to be used as a quality control tool. As was described earlier, when we mix these investments, we are setting up a distribution of the particles in the liquid. This process can be followed by mixing the material for a given period of time and then measuring the Zahn Cup flow time. The graphs below (Figs. 2 and 3) show the type of data which one can obtain.
| Fig. 2: Effect of L/P Ratio on Fluidity (mixing time 11 minutes) |
| Fig. 3: Effect of mixing time on fluidity (L/P ratio = 34) |
They are for a type A material which is recommended to be used at 34/100 with a mixing time of 11 minutes. These conditions had been determined several years ago by the trial and error method of simply looking at the mix, but they are close to the ones which the Zahn Cup testing would suggest. Earlier in this discussion I had mentioned that in this class of material the additive used as a sintering agent also acts as a fluidizing agent. Figure 4 shows the effect of varying the amount of the sintering agent on the Zahn Cup time.
| Fig. 4: Effect of additive level on fluidity |
While low levels of the agent give good fluidity, they are not necessarily high enough to give the required sintering reaction.
The Type A and Type B materials can be made using the same type of sand in theory, the only difference being the use of a small amount of acid. One way to use the Zahn Cup test as a Q.A. tool of the quality of the sand being used is to carry out the test with two different mixing times, the shorter one being less than the recommended and the longer one being the recommended mixing time. Table 2 shows data on several codes of the same sand when used in a type A and an experimental type B formulation.
| Table 2: Effect of particle size on fluidity |
| Table 3: Casting results for various sands |
In the A formulation, the codes, with the exception of O, behave identically within experimental error. While in the B formulation, there is considerable difference and that this can be tied to the particle size as defined by the percent of 10 micron particles. It is data of this type which allows us to specify the sands that we use.
When we first started to work on a Type C material we looked at a large range of sands. We wanted to use a finer sand than in the A or B type material because we thought it was necessary to give a smoother surface. Going back to the gypsum bonded materials, the binder fills in the gaps between the sand particles and thus can give a smooth surface; while in the platinum investment material we have to rely on the sand particles themselves with the smaller ones filling in the gaps between the larger ones. We collected samples of all the readily available sands which were as fine as or finer than the ones used in A and B types and tried to use them with a phosphoric acid based liquid.
We found that there were considerable differences in behavior which seemed to relate more to the origin of the sand than to the properties which we could easily measure. If we tried to use two sands from the same facility, the difference which we could measure did make sense in terms of the behavior of the investment. To say a little about what I have been calling sands, they are more correctly called silica flowers. They are produced by mining quartz sand from a sand pit, cleaning it up and grinding it. What is actually extracted from the ground is a mixture of sand, clay and whatever else mother nature deposited there. The relatively coarse sand particles are extracted from this. In some cases this can be achieved by floatation techniques, but in others, acid treatments are necessary to get rid of contaminants. Once the pure sand particles are obtained, they are ground to the required size using continuous ball mills. The coarse sand is fed in one end and the required silica flower comes out of the other. Particle size is controlled by how long it takes the material to pass through the mill. The specific shape of the particles is controlled in a large part by how the larger particles break up and this goes back in part to how they were originally formed.
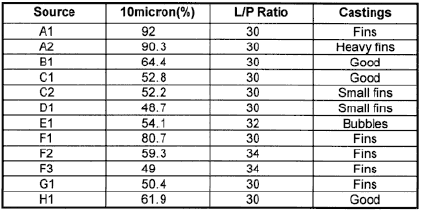
The first letter denotes which facility was the source of the sand. We found that if we needed to use liquid/powder ratios greater than 30/100 we generally ran into strength problems. The same was true if the particle size was too fine. This was primarily based on our in-house casting tests. We then selected two materials to field testing. They used sands with similar production processes and particles sizes but from geographically different locations. The liquid used was based on Phosphoric acid. One sand proved to give slightly better surfaces than the other, and I will present data on it later.
| Table 4: Physical properties of various commercial investments |
Properties of Investments
Listed in Table 4 are the properties that we have measured on various commercial investments including our own. The letter designation goes with the A,B,C system that I used earlier. The materials were mixed according to the manufacturers instructions. A and B use water while the others use Phosphoric acid-based liquids. We are still trying la understand all the meanings of this data. The liquids used with C1-C4 are all different and certainly are important in controlling properties. C1 and C2 have the finest particle size and the lowest permeabilities. From our field testing the permeability is sufficient and the finer particle size gives smoother surfaces. We do think that there is a relationship between particle size and these properties.
The strength numbers need more work before we can claim to understand them. The fact that some materials were stronger when burnt out at 1400°F than at 1600°F surprised us, but we know that some people use 1400°F in preference to 1600°F while others use even higher temperatures. It would be interesting to be able to actually test these materials at the burnout temperatures as cooling them to room temperature may not give the full picture. All materials are significantly stronger than the Gypsum bonded investments however and this was one of the requirements discussed earlier.
Fluidity is an important property and we believe that it should not be too long but we can't say what is acceptable and what is not. It depends on the material and how it is being used. A major area of use of this general type of investments is the casting of certain medical and dental parts. These are relatively high volume applications and they use semiautomatic equipment which invests multiple flasks from the same large mix. Fluid investments are important to them, as they hopefully will be to the Platinum casting industry.
Finally, thermal expansion. All the listed materials are fairly similar. Even though they do not contain cristobalite, they have higher expansions than gypsum investments.
Use of the Investment
The first important step in using these investments is to use them at the correct liquid/powder ratio and to mix them long enough. This is necessary to achieve the required structure in the slurry. If this is not achieved, then it will have a negative effect on both the strength of the investment and on the surface quality of the casting. We know that larger mixes take a longer mixing time than smaller ones from our measurements of fluidity and also from problems encountered by people who use this type of investment to cast medical and dental devices out of super alloys and who have scaled up the size of their mix.
The slurry should be well vacuumed. In general, there is not a problem of air bubbles being trapped on the surface of the patterns as these investments wet the patterns well, but if air is trapped in the investment, it can weaken it.
The bench setting of these investments is very important. The material forms a structure as it sets. I don't claim to fully understand what is happening but it is likely that a silica gel is being formed. Silica gels are complex materials and there is a lot of technical and scientific literature on them. They form over a period of time and how they form will be dependent on the chemistry within the slurry. As water is removed from the setting investment, the formation of the gel is sped up. The structure of the gel will be a function of how rapidly it is formed, and therefore the behavior of the investment will be a function of the drying rate. Faster is not necessarily better. Different users have different methods to remove water from the setting investment and this is one area where variations in technique can give variable results.
To a greater or smaller extent, some settling of the investment occurs during setting. In an ideal situation this would not affect the structure of the interface between the pattern and the investment but we know that it can. This particularly can happen in the older types of investment which are used at higher water/powder ratios if they are not wellmixed. It should not happen with a well formulated material used at 30/100, if it is used correctly. What occurs is that a water rich layer is formed on the underside of the pattern and this results in a poor surface.
During burnout, care has to be taken not to heat the investment too rapidly at lower temperatures when the wax is being be removed and the excess water driven off. This is no different than with a gypsum investment. From the thermal expansion curve we know that the expansion, as a function of temperature, is relatively smooth compared with gypsum investments. This means that the expansion behavior of the material should not limit the rate at which it can be heated. One topic that we have not yet evaluated is how the strength of the Investment varies with temperature as it is burnt out. This will be interesting to know, as it could influence the way in which the investment should be burnt out.
There is a lot that we don't know or fully understand about these materials and we continue to work on them. Hopefully we will be able to continue to improve them. A very important part of the effort is the feedback that we get from field testers and other users. In closing I would like to particularly thank those of you out there who have worked with us.
You assume all responsibility and risk for the use of the safety resources available on or through this web page. The International Gem Society LLC does not assume any liability for the materials, information and opinions provided on, or available through, this web page. No advice or information provided by this website shall create any warranty. Reliance on such advice, information or the content of this web page is solely at your own risk, including without limitation any safety guidelines, resources or precautions, or any other information related to safety that may be available on or through this web page. The International Gem Society LLC disclaims any liability for injury, death or damages resulting from the use thereof.
The All-In-One Jewelry Making Solution At Your Fingertips
When you join the Ganoksin community, you get the tools you need to take your work to the next level.
Trusted Jewelry Making Information & Techniques
Sign up to receive the latest articles, techniques, and inspirations with our free newsletter.